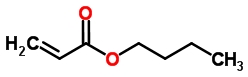
Description
Butyl acrylate is produced by reacting butanol with acrylic acid in the presence of an acid catalyst at an elevated temperature to produce butyl acrylate, water and other by-products. The product mixture is purified by distillation.
Chemical & Physical Properties
| Density |
0.9±0.1 g/cm3 |
| Boiling Point |
145.9±9.0 °C at 760 mmHg |
| Melting Point |
-69 °C |
| Molecular Formula |
C7H12O2 |
| Molecular Weight |
128.169 |
| Flash Point |
39.4±0.0 °C |
| Exact Mass |
128.083725 |
| PSA |
26.30000 |
| LogP |
2.39 |
| Vapour density |
>1 (vs air) |
| Vapour Pressure |
4.8±0.3 mmHg at 25°C |
| Index of Refraction |
1.418 |
| Stability |
Stable. Flammable. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| Water Solubility |
1.4 g/L (20 ºC) |
Uses
Butyl acrylate is primarily used in the production of homo and co-polymers emulsion for use in water based architectural and industrial paints. Polymers with butyl acrylate can also be used in manufacturing cleaning products, leathers industries, antioxidant agents, plastics, enamels, inks, adhesives, sealants, textiles, caulks and paper finishes. The acrylate functionality allows it to be used as a chemical intermediary.
| Storage and Shipping Information |
| Storage |
Store below +30°C. |